
Volume 83, Number 2, 2005
One of These Things Is Not Like the Others:
The Idea of Precedence in Health Technology
Assessment and Coverage Decisions
Mita Giacomini
McMaster University
Health plans often deliberate covering technologies with challenging purposes, effects, or costs. They must integrate quantitative evidence (e.g., how well a technology works) with qualitative, normative assessments (e.g., whether it works well enough for a worthwhile purpose). Arguments from analogy and precedent help integrate these criteria and establish standards for their policy application. Examples of arguments are described for three technologies (ICSI, genetic tests, and Viagra). Drawing lessons from law, ethics, philosophy, and the social sciences, a framework is developed for case-based evaluation of new technologies. The decision-making cycle includes (1) taking stock of past decisions and formulating precedents, (2) deciding new cases, and (3) assimilating decisions into the case history and evaluation framework. Each stage requires distinctive decision maker roles, information, and methods.
Key Words: Technology assessment, ethics, resource allocation, social values.
One of these things is not like the others,
One of these things just doesn’t belong.
Can you tell which thing is not like the others
By the time we finish our song?
—Kermit the Frog, Sesame Street
![]() ealth systems routinely face the problem of
deciding whether to publicly insure novel emerging health
technologies. Recent challenging technologies include, for
example, new reproductive techniques such as intracytoplasmic sperm
injection in vitro fertilization (ICSI IVF), new molecular genetic predictive
tests for hereditary breast cancer (e.g., the BRCA1/2 test), and
new drugs such as sildenafil (Viagra) for sexual dysfunction. Novel technologies
often raise ethical, legal, and social concerns about their role
and value in the health care system. These concerns transcend the traditional
questions about safety, effectiveness, and affordability. When
confronted with disorienting new health care technologies, we often describe
them as “unprecedented.” Yet deliberations about their place in
the health care system frequently turn to the idea of precedent: the question
of how a new technology, of which we are skeptical, compares with
established technologies, to which we are clearly committed. If the new
one is enough like the already covered ones, this intuitively favors its
inclusion as a covered benefit. If not, the technology may be excluded
from coverage until a stronger argument for its value prevails. Decision
makers often also consider whether today’s coverage decisions establish
“precedents” for the coverage of tomorrow’s new technologies or cast
doubts on yesterday’s coverage decisions. When we cover ICSI, a genetic
test for breast cancer genes, or Viagra, does this oblige us to cover other
infertility technologies, all genetic tests, or other “lifestyle” remedies?
If we do not cover them, should we, to be fair, remove similar services
from the list?
ealth systems routinely face the problem of
deciding whether to publicly insure novel emerging health
technologies. Recent challenging technologies include, for
example, new reproductive techniques such as intracytoplasmic sperm
injection in vitro fertilization (ICSI IVF), new molecular genetic predictive
tests for hereditary breast cancer (e.g., the BRCA1/2 test), and
new drugs such as sildenafil (Viagra) for sexual dysfunction. Novel technologies
often raise ethical, legal, and social concerns about their role
and value in the health care system. These concerns transcend the traditional
questions about safety, effectiveness, and affordability. When
confronted with disorienting new health care technologies, we often describe
them as “unprecedented.” Yet deliberations about their place in
the health care system frequently turn to the idea of precedent: the question
of how a new technology, of which we are skeptical, compares with
established technologies, to which we are clearly committed. If the new
one is enough like the already covered ones, this intuitively favors its
inclusion as a covered benefit. If not, the technology may be excluded
from coverage until a stronger argument for its value prevails. Decision
makers often also consider whether today’s coverage decisions establish
“precedents” for the coverage of tomorrow’s new technologies or cast
doubts on yesterday’s coverage decisions. When we cover ICSI, a genetic
test for breast cancer genes, or Viagra, does this oblige us to cover other
infertility technologies, all genetic tests, or other “lifestyle” remedies?
If we do not cover them, should we, to be fair, remove similar services
from the list?
But what does it mean analytically for a health care technology to be precedented or to set a precedent for future decisions? Precedent has a variety of lay, legal, and policy meanings. The concept of precedent, case-based reasoning, and analogical methods for comparing and evaluating health technologies have much to offer the field of health technology assessment and coverage policymaking. I pursue several aims in this article. First, I examine why analogical reasoning seems necessary in evidence-based coverage decision making, particularly for determining standards and assessing the qualitative dimensions of new technologies. Second, I demonstrate how arguments from precedent have already arisen implicitly in current deliberations over the value of new technologies. I use legal and economic discourses concerning three controversial technologies (ICSI, genetic predictive tests, and Viagra) to illustrate this phenomenon. Finally, I consider how analogical reasoning and arguments from precedent might be employed more consciously, systematically, and critically. The literatures of several disciplines (philosophy, psychology, political science, law, and ethics) offer helpful lessons regarding the arguments from analogy and precedent in the development of policy. From these, we can begin to characterize the basic features of a case-based, precedent-conscious reasoning and decision cycle and to adapt this logic specifically to the problems of technology assessment and coverage decision making. The resulting framework offers a way of meshing the ethical evaluation of health technologies with their instrumental evaluation and also of systematically developing fairer standards by which to judge emerging health services.
Criteria for Evaluation and the Need for Standards
It is widely accepted that an emerging health technology must meet certain criteria to qualify for public insurance coverage. “Evidence-based decision making” relies on information regarding a technology’s performance on each of these criteria. The wide range of potential coverage criteria has been reviewed elsewhere (Giacomini, Miller, and Browman 2002; Hurley et al. 2000). All publicly funded health insurance programs consider safety and effectiveness, and some consider cost (unit or aggregate), demand, or cost-effectiveness. In addition, a number of qualitative criteria are important. “Medical necessity” is a basic requirement in many jurisdictions, including Canada, where this criterion is federally legislated by the Canada Health Act. A number of informal quasi criteria have emerged in efforts to clarify what is not medically necessary, for example, the exclusion of “lifestyle,” “elective,” or “cosmetic” services. Other examples of proposed qualitative criteria are “ethics” (Wilson, Rowan, and Henderson 1995), individual responsibility (Government Committee on Choices in Health Care 1992), solidarity (Calltorp 1999), and social or individual wants (Deber, Ross, and Catz 1994). In an assessment and coverage model developed for the Ontario Provincial Advisory Committee on New Predictive Genetic Technologies, we proposed six criteria for consideration: intended purpose, effectiveness, additional effects, cost, demand, and cost-effectiveness (Giacomini, Miller, and Browman 2002). The criteria and concepts are described in their most basic terms in Table 1.
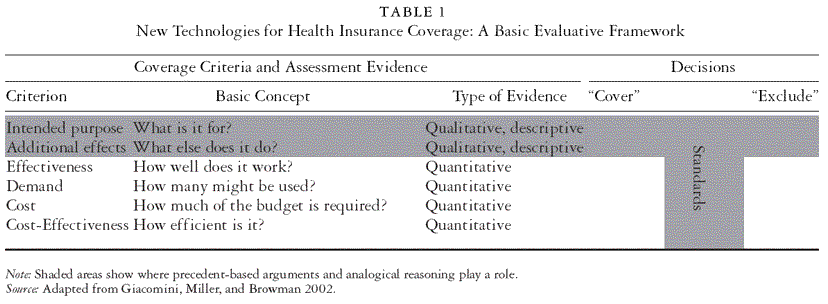
Coverage decision making is also becoming more participatory, with the energetic promotion (although piecemeal uptake) of models such as Daniels and Sabin’s “accountability for reasonableness” (Daniels and Sabin 1997, 2002; Hansson, Norheim, and Ruyter 1994). Such deliberative models require decision processes to be transparent (i.e., the decisions and their rationales must be made available to the public), based on relevant principles (i.e., criteria such as effectiveness and efficiency), appealable, and regulated to ensure that accountability features are enforced. Recent national health care reform proposals in Canada emphasize greater accountability through citizen involvement in “core service” definition or coverage decisions (Commission on the Future of Health Care in Canada 2002; Senate Standing Committee on Social Affairs Science and Technology 2002).
Scholars of technology assessment and resource allocation have extensively devised methods for generating evidence, operationalized important evaluation criteria such as effectiveness and efficiency, and prescribed fair decision processes for which evaluative evidence may be applied. But the technology assessment community has given far less attention to the problem of operationalizing for decision makers essentially qualitative and normative criteria such as whether the technology serves an “ethical” or “medically necessary” purpose and which of its effects might be good or bad. The first two criteria in Table 1, “intended purpose” and “additional effects,” are qualitative criteria in that they require descriptive evidence for understanding what the technology is and does, rather than quantitative evidence of how much it does or costs. These descriptive questions are fundamental to any further technology assessment; it is pointless to ask means-oriented questions about effectiveness or efficiency before the ends have been identified. Also relatively neglected is the crucial task of establishing the standards by which decision makers might judge the evidence when applying the criteria: how good is good enough? The place of standards is indicated in Table 1 by the gray-shaded area between coverage and exclusion decisions; the problem of setting standards applies to quantitative and qualitative criteria alike. For qualitative criteria, the standard-setting question is which categories are acceptable, and the technology assessment question asks whether a specific technology falls into an acceptable category. For the quantitative criteria (the traditional domains of “evidence-based decision making”), the standard-setting question concerns the specific performance thresholds (or cost ceilings), while technology assessment is concerned with whether a given technology meets them (or exceeds them).
In summary, even in the context of fair process and evidence-based reckoning, each coverage decision-making body faces afresh the problem of how to draw the line between the worthwhile and not worthwhile, effective and ineffective, and efficient and inefficient. The evidence may suggest that a new test yields three additional years of life, that an infertility procedure yields one genetically related baby for every four interventions, or that a treatment for sexual dysfunction works 80 percent of the time. But evidence cannot establish whether three years, one baby, or four tries is a lot, a little, too much, or enough. Evidence also cannot indicate whether genetic relatedness is a valid health care goal or whether sexual function qualifies as a medical necessity. Without such standards, evidence takes us nowhere nearer to decisions, and evidence- and criteria-based coverage frameworks lose their power. Without a method for establishing standards, decision makers either struggle to reinvent these wheels again and again or rely on vaguely defensible “rules of thumb” (such as the oft-touted but seldom explained $50,000 per quality-adjusted life year (QALY) standard for cost-effectiveness in health services). Limited attempts to derive standards deductively, for example, the quest for a dollars-per-QALY standard from studies of human capital and willingness to pay, have failed to produce a consensus (values range from U.S. $24,977 to U.S. $428,286 per QALY) (Hirth et al. 2000).
Where else might standards come from? Decision makers often grope around for precedents with which to compare the unprecedented, taking account of selected aspects of a new technology that are arguably familiar. If they can find a robustly analogous technology, this discovery will favor coverage if the analogous technology does not differ substantively from the new technology. Deliberations about what constitutes a substantive difference, in turn, offer opportunities to clarify and reconcile the diverse criteria by which we value new health care technologies.
Examples
Analogical reasoning is part of the deliberations regarding the coverage of three health care technologies whose legitimacy as insured services has been vigorously debated: ICSI, Viagra, and predictive genetic tests. The following are some examples of how precedents have been invoked to address the problem of establishing standards for two particularly important but problematic coverage criteria: acceptable purposes (i.e., whether the technology is a medical necessity or otherwise serves a legitimate purpose as a collectively funded health service) and acceptable cost or cost-effectiveness (i.e., affordability and value for money).
ICSI
ICSI is a variation of in vitro fertilization that addresses certain types
of male infertility. In the procedure, a sperm that might not manage
its way into an egg is microscopically inserted into the egg to achieve
conception. The fertilized egg is then implanted into the female partner
in hopes of achieving a pregnancy. Controversial features of ICSI include
its high unit cost, its gendered nature (e.g., as a treatment for “male”
infertility), and the variety of objectives it pursues (e.g., the achievement
of children genetically related to their parents, as well as conception,
pregnancy, and babies generally). ICSI is one of few technology coverage
decisions to be challenged in court in Canada, in Ontario in 1998 (D.R.
and L.R., B.C. and L.A.C., B.L. and R.F., L.E. and M.E., J.H. and K.H.
v. Ontario 1998) and in Nova Scotia in 1999 (Cameron and Smith v. Nova
Scotia 1999). Both provincial health plans had refused to cover ICSI as
an insured service. Both appeals were dismissed, and in both provinces
ICSI remains a service not publicly insured. Between the two cases, many
“precedent” services—both covered and not covered—were invoked to
characterize ICSI’s purpose as a medical necessity (or not) and cost or
cost-effectiveness as acceptable (or not) (see Table 2).
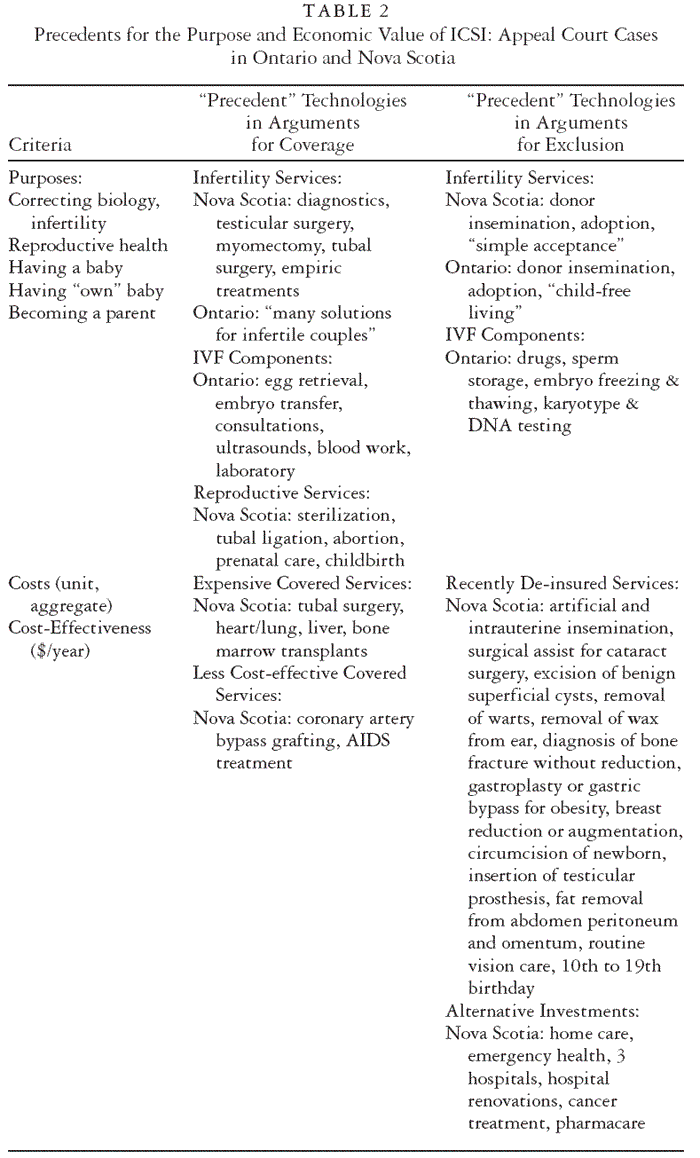
In both provinces, the issue arose of whether infertility services as a class belonged in the public health insurance plan. Advocates for coverage in Nova Scotia pointed out that diagnostics, testicular and tubal surgery, and other infertility interventions were paid for. In Ontario, an even stronger precedent was the coverage of in vitro fertilization (IVF), which is closely clinically related to ICSI. Indeed, IVF is necessary for ICSI, leaving Nova Scotia obligated to cover IVF in the event of a decision to cover ICSI. Both provinces countered that other infertility services, for example, donor insemination, are not publicly insured. This comparison caused the rationales to be questioned, for example, the idea that “medical” entails correcting biology, not simply achieving a pregnancy or parenthood. It was noted that neither government covered the costs of adoption, and in Ontario it was also argued that “child-free living” is a nonmedical alternative to ICSI. In Ontario, because IVF is covered, ICSI was portrayed as a component of IVF, and the argument was made that other IVF components shared with ICSI were covered health services: egg retrieval, ultrasounds, embryo transfer, and the like. The province countered that other IVF components are not covered: drugs, sperm storage, and so forth. Finally, in Nova Scotia, ICSI was characterized by its advocates not just as an infertility service but also as a reproductive health service: appellants asked why, if medicare covers surgical sterilization, abortion, prenatal care, and childbirth, it would not cover infertility care too?
The ICSI procedure is fairly costly, the level of demand is unknown, and its cost-effectiveness has not been well evaluated. Particularly in Nova Scotia, economic concerns figured prominently in the arguments. In the hearing, experts suggested that every episode of ICSI would cost the province roughly $3,500 and that every ICSI-produced “life year” cost about $145. To put these numbers in perspective, ICSI advocates pointed out that the province covers many more expensive services, including tubal surgery (for infertility) and several types of organ transplant: heart/lung, liver, and bone marrow among them. In addition, the appellants argued that the province covers less cost-effective services, offering cardiac bypass surgery and treatment for AIDS as cases in point. The province contended that cost control was a legitimate policy objective and that ICSI was not being singled out for exclusion; many services had recently been removed from the list (see Table 2) and the savings had been reinvested in needs more pressing than infertility, like home care, emergency services, and hospital renovations.
Viagra
Viagra is a well-publicized pharmaceutical treatment for erectile dysfunction.
It is controversial for its potentially high aggregate cost if
widely marketed and utilized, its potential for abuse (i.e., by people without
dysfunction seeking enhanced sexual experiences), and its “lifestyle”-serving nature (i.e., the remedy of sexual problems is debatable as a medical
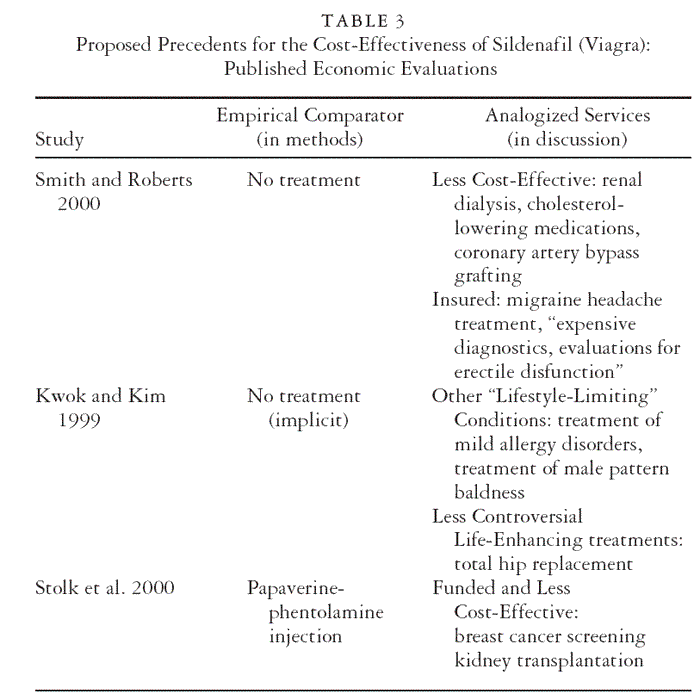
necessity). Table 3 summarizes the comparisons of technologies made
in published cost-effectiveness evaluations of Viagra to date. The second
column lists the comparators used for the empirical economic evaluation,
the services against which Viagra’s marginal costs and effects were
actually measured, that is, either no treatment or an injection for erectile
dysfunction. However, much broader analogies to other services—which
were not addressed empirically—arise in the reports’ discussion sections,
in which the authors strive to put their findings into perspective and
often make precedent-based arguments for the acceptability of the evaluated
technology. The third column of Table 3 lists those services that are
invoked in this way. Smith and Roberts (2000) and Stolk and colleagues
(2000) argue that less cost-effective services are covered: renal dialysis,
cholesterol medications, bypass surgery, breast cancer screening, and kidney
transplants. This invites questions, of course, about whether there
is something missing in a metric (dollars per QALY) that precludes distinctions
between the purposes of achieving an erection and treating end
organ failure or preventing cancer. Kwok and Kim (1999) point out that
less controversial services aimed to improve the quality (not length) of
life, like total hip replacements, are insured. In response to the popular
intuition that Viagra is a “lifestyle” technology, as opposed to a medical
necessity, arguments are made both for and against its coverage.
Smith and Roberts (2000) point out that we do cover life-enhancing
technologies, including diagnostic services for erectile dysfunction and
the treatment of migraine headaches. Kwok and Kim (1999) caution
that covering Viagra establishes the context for covering treatments for
other “lifestyle-limiting” conditions, including mild allergies or male
pattern baldness.
Analogies to Viagra become even livelier in published analyses of the coverage quandaries faced by health plans at the time of the drug’s appearance on the market. One author determines that “evidence-based” decisions would favor Viagra (proven effective) over antibiotics for throat and chest infections (unproven) (Haslam 1998). Another argues that a commitment to improving quality of life (rather than longevity) is embodied in decisions to cover, for example, treatments for migraines, arthritis, and stroke (Keith 2000). The nature and legitimacy of “lifestyle” technologies is critiqued through comparisons with weight loss drugs (Atkinson 2002), hair restoration (Atkinson 2002), antidepressants (Atkinson 2002), infertility treatments (Daniels, Teagarden, and Sabin 2003), contraceptives (Daniels, Teagarden, and Sabin 2003; Phillips, Spetz, and Haas 2003), and cosmetic surgery (Keith 2000). These issues are further complicated by the fact that a life-saving leukemia drug is now in demand for its lifestyle-enhancing ability to restore color to gray hair (Atkinson 2002) and the suggestion that Viagra, like antidepressants, generates happier employees and thus favorable effects on the economy (Parens 1998).
Predictive Genetic Tests
Predictive genetic tests seek molecular genetic anomalies associated with genetically mediated diseases. They generate risk information, which may or may not be acted on to prevent or detect disease. These tests are controversial for their widely varying clinical utility, the potentially high aggregate cost of testing and follow-up care (especially with vigorous marketing and induced demand), and societal implications for understanding, experiencing, and acting on genetic risks.
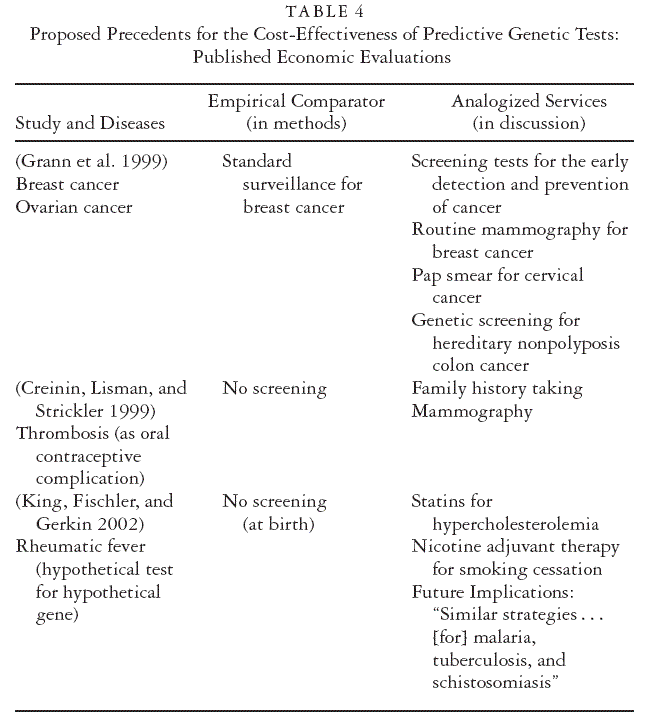
Table 4 summarizes the technologies that the authors of economic
evaluations have used to assess the emerging predictive genetic tests versus
to put their findings into context. The studies cover tests for a range
of genetic defects and associated diseases (including a hypothetical test
for an undiscovered gene) (King, Fischler, and Gerkin 2002). Empirical
comparators are either no genetic screening at all, conventional forms of
genetic screening, or early disease surveillance and detection. In summative
discussions, however, these cost-effectiveness studies compare
predictive genetic tests favorably with other screening tests for similar
diseases (cancer, congenital) as well as with diagnostic tests, which find
disease rather than predict it (including mammography and pap smears).
Comparisons with mammography may be intuitively sensible in the case
of genetic tests for breast cancer because they address a similar disease
(albeit diagnostically, not predictively). But mammography also is invoked
by studies of genetic tests for cystic fibrosis and predisposition
to thrombosis. The study of a nonexistent test for a yet-undiscovered
gene for a predisposition to rheumatic fever favorably compares the
cost-effectiveness of this “test” with pharmaceutical treatments for high
cholesterol and smoking cessation. It is argued to set a positive precedent
for the future development and coverage of “similar strategies” for
malaria, tuberculosis, and schistosomiasis (King, Fischler, and Gerkin
2002). Similarly, a test for cystic fibrosis is characterized as setting the
stage for future technologies such as gene therapy (Wildhagen et al.
1998).
The Method in the Muddle
Multidisciplinary Perspectives on Analogy and Precedent
Although we have methods for generating good evidence, we lack standards for judging the purposes of technologies, as well as impacts such as effectiveness, costs, and cost-effectiveness. In actual coverage arguments, most deliberators—from litigators to evaluators to activists—muddle through by intuitively invoking “precedent” health technologies that have been either covered or excluded in the past. Selected technologies, such as mammography or renal dialysis, emerge as implicit standards for a variety of evaluative criteria, not only cost-effectiveness (Hirth et al. 2000), but also worthy target diseases, populations, and other qualitative concerns. Arguments in favor of technologies as diverse as predictive genetic tests and Viagra hinge on their likeness to mammography, currently a well-accepted, funded, and promoted technology. Arguments by analogy also help reveal conflicts among evaluative criteria such as purpose, effectiveness, equity, and cost-effectiveness. For example, while Viagra and mammography may be comparable in cost-effectiveness, it is their qualitative difference in purpose that keeps many from accepting Viagra as simply a “me-too” method for producing QALYs, as its champions argue we should (Kwok and Kim 1999). In these ways, evaluation by analogy supplements and enhances, rather than replaces, conventional criteria and evidence as we judge the value, legitimacy, and place of emerging health technologies.
Apparently, analogy and precedent-based argument have become widespread but largely unexamined methods for assessing technologies. The logic of precedence occasionally arises explicitly when assessing conventional technologies, for example, to grandfather devices for premarket approval under the regulations of the U.S. Food & Drug Act, section 510K, or, more generally, to identify “me-too drugs.” However, more implicit arguments from analogy and precedent also play a powerful role in the evaluation of unconventional new technologies. Such arguments can be pivotal in difficult coverage deliberations. In particular, they help identify, interpret, and apply evaluative criteria, as well as identify and apply relevant technology assessment evidence. This intuitive, case-based style of reckoning might be examined more closely for its potential as a formalized and methodical approach to the evaluation of emerging health technologies. For this, we can draw guidance from several disciplines in which analogical reasoning and precedent-based arguments are of central interest: law, ethics, philosophy, psychology, and political science.
Is it possible for analogizing to be rigorous or robust, as evaluative methods presumably ought to be? Philosophers caution that as a method of logic, analogical reasoning is never airtight, but analogical arguments may be constructed in more or less cogent and compelling ways (Copi 1986; Hitchcock 1992). Analogical inference leads to conclusions that are presumptive, not certain. In the case of causal arguments, analogies are used to generalize probabilistically from past cases to a slightly different present case on the basis of key similarities (Copi 1986). In the case of moral arguments, analogies are used to classify descriptively rather than to predict probabilistically. They thus rely on—and, in turn, refine—the essential constitutive criteria that associate the case in point with the analogized case (Hitchcock 1992). For either analytic aim, analogical arguments and conclusions are sound to the extent that they are reasonably believable for the present purpose, but they remain open to revision. The aim of analogizing is not to identify the single correct analogy, because there is no such thing. Rather, the aim is to argue over—and agree on—the most analytically compelling analogies, of which there may be many or few. The complementary analytic task is to dispose of poorly substantiated or irrelevant analogies, of which there are multitudes. Of the criteria for evaluating analogy, the relevance—not the number—of the features highlighted by the comparison is crucial, as is, of course, their fit between the case in point and the analogized case (Copi 1986). In the context of policy, the relevance of features may be highly disputable. The process of settling on appropriate analogies (e.g., total hip replacement as an analogy for Viagra) thus involves settling on legitimate criteria for coverage decision making (e.g., comparable cost-effectiveness may be trumped by widely divergent clinical purposes or target populations, to which the latter are an important criterion for coverage policy decisions).
Despite the leaky logic of analogy, cognition experts suggest that we must make analogies in order to learn (Hofstadter 1996a; Holyoak and Thagard 1995). If we cannot categorize familiar phenomena and skillfully and creatively fit a new phenomenon within the context of what is already known, we cannot learn. This applies to people and computers alike, and arguably it applies to the institutions that make health care coverage decisions. Furthermore, as a species of metaphor, analogy also allows us to imagine possibilities and alternatives. Metaphor grants us the power to relate what is to what might be and plays a well-recognized role in political rationality and policymaking (Schlesinger and Lau 2000; Stone 1998). The key skill of the creative imagination is to find the conceptual “knobs” on familiar things, “tweak” them, and watch the transformation of one thing into something else (Hofstadter 1996b). By definition, novel technologies require this sort of imagination to evaluate wisely. Fiddling with novel features and making new technologies seem more and less like the ones we know help clarify our concerns, values, hopes, and fears about them. What if ICSI were relatively inexpensive, like ovulatory drugs, or entailed fewer health risks, like artificial insemination? What if predictive genetic tests or Viagra became available over the counter, like pregnancy tests? What if excluding any of these things from coverage led research and development back to the drawing board to come up with something more acceptable—what might this alternative be like? And so forth.
Such analogizing often points us to precedents, either technologies past or technologies to come as a consequence of current decisions. Precedent-based reasoning, or the doctrine of stare decisis, has long formed the backbone of common law. Legal scholars have suggested that a generalized model of precedent-based reasoning may be fruitfully adapted from common law to other policy spheres (Schauer 1987). In health policy, advocates of fair and transparent coverage decision making also point to common law as a model for fair case-by-case decision making about new health services (Arras 1991; Daniels and Sabin 1997). From the perspective of political science, Aronovitch (1997) encourages analogy-based policy analysis in part because policymaking is necessarily incremental. We may be able to envision a perfect world, but we cannot get there from here in a single leap. Analogizing, and particularly arguments from precedent, can be used to reveal a feasible path to reform, which can be trod one step at a time: “Analogical argument is eminently apt for politics because analogy and politics both involve building innovatively upon the past by constructively extending precedent. In that way stability, agreement, and progressive steps into the future are secured” (Aronovitch 1997, 78).
Legal scholars caution, however, that while incremental policymaking is relatively feasible, it also is concerned with myopic, immediate problems rather than with the bigger, long-term view (Eisenhower 1988) or the integrity and coherence of the policy system as a whole (Sunstein 1993). Precedent-based arguments tell us more about how a new technology “fits” into the status quo than how the health system should look in a decade. Precedent-based decisions are like policy spackle: best applied to gaps and rough edges. Their extensive accumulation will not necessarily produce a sturdy, elegant framework. There must be occasions for grand redesign based on fundamental goals (e.g., national health reform), and on these occasions, badly fitting precedent-based decisions may be rightfully ignored or overturned.
There are other lessons to be learned from experience with precedent in common law. The first is that precedent may either amplify or attenuate ideological biases (Banks 1999). If we distrust the biases of past decision makers, precedent-based policymaking will be unappealing because of its tendency to perpetuate those biases. Precedent-conscious decision making tends to have a conservative effect and to work against reform; however, it also works against capricious meddling (Banks 1999; Fletcher 1988). If we distrust more the biases of current decision makers, holding them to precedents will tend to constrain them, mold their thinking, and protect a more balanced policy legacy (Akanmidu 2001). Second, scholars debate the usefulness of analogical reasoning in forging morally acceptable decisions in pluralistic communities. For example, Sunstein (1993) suggests that it is easier for ideologically diverse parties to agree on compelling analogies than on fundamental principles; thus it is more expedient to justify decisions with arguments from analogy than with arguments from principle. But bioethicists like Wildes (2000) insist that analogies appear compelling only against a backdrop of some minimally shared values. Analogical arguments cannot gloss over deep moral rifts, and if the rifts are too wide, compelling analogies cannot be made at all. Third, while argument from precedent and analogy can be an efficient way of making a point, once a compelling analogy has been made, it may become easy to overlook important particulars of the case that are not well represented in the analogy and to neglect other issues worthy of attention (Rifkin 2001; Schauer 1987). We see this struggle in health policy when league tables (ranking health services by dollars per unit of health produced) are offered with the suggestion that cost-effectiveness should be the sole feature by which health services are compared. If followed, this approach forecloses consideration of other crucial evaluative criteria such as the legitimacy of the technology’s purposes and effects, populations served, and budgetary affordability. Yet, rejoining analogies can be the antidote to overly narrow evaluative comparisons:
A special advantage of analogical reasoning over economic analysis is that the former, unlike the latter, need not insist that plural and diverse social goods should be assessed according to the same metric. To make diverse goods commensurate in this way may do violence to our considered judgments about how each good should be characterized. (Sunstein 1993, 787)
Fourth and finally, wise decision making entails more than analogical argument, and analogizing is a learned skill that may be done well or poorly (Rifkin 2001).
Casuistry
One school of bioethics, casuistic analysis, offers a methodology for precedent-conscious, case-based decision making. Casuistry is a method of moral evaluation with ancient philosophical and theological roots (Jonsen and Toulmin 1988a; Miller 1996a). It was most notoriously developed by 16th- and 17th-century Jesuits to diagnose the sins of confessors, assess their gravity, and assign proportionate penance. Nuanced moral sensitivity was exercised in regard to the particularities of cases; extenuating circumstances could alter both the class and the weight—and thus the consequences—of a sin. Casuistry also exploited and enlarged loopholes in the fabric of moral reasoning. Absolution often required sophisticated interpretation of extenuating circumstances, leaving penitents at the mercy of their confessors’ analogical reasoning skills. Casuistry eventually became discredited as a method of “scholastic sophistry in the service of moral mediocrity” (Miller 1996b, 4), and the lay term casuistry retains this negative connotation today. However, contemporary bioethicists have revived formalized casuistry as a respectable—and even necessary—method of applied moral reasoning. The place of casuistry in bioethics has been addressed elsewhere (Arras 1991; Jonsen and Toulmin 1988a; Miller 1996a; Wildes 2000). Note, though, that casuistry supplements rather than replaces conventional principle-based bioethical reasoning (analyzing ethical dilemmas in terms of conflicts among principles such as beneficence and justice) ( Jonsen 1995a; Wildes 2000). The structure of casuistic ethical analysis offers some useful elements that might be adapted to a more formal model of precedent-based technology assessment and coverage decision making.
A fundamental problem in principle-based ethical reasoning is how to map particular cases to general principles. A truly basic ethical question is not “Is justice more important in this case than nonmaleficence?” but “Is this a case of ‘injustice’. . . or is it essentially something else?” Casuistic reasoning is used to “diagnose” the nature and gravity of an ethical issue. Diagnosis, in turn, points us to the relevant principles and leads to a judgment based on how we have previously resolved similar ethical issues, appreciating that general principles may need to be adapted to particular novelties in the current circumstance ( Jonsen and Toulmin 1988b). Judgment must be decisive enough to support action yet remain presumptive and revisable: “[There is] methodological kinship [of casuistry] to the physician’s ways of analyzing and resolving medical problems of clinical diagnosis. . . . In morals and medicine alike, the most confident opinion about a particular case may subsequently be called in question in the light of fresh considerations” ( Jonsen and Toulmin 1988b, 257). Casuistry—or case-based ethical reasoning—plays out dialectically, with each step in the cycle affecting subsequent ones. The ethicist must be familiar with a case history of paradigmatic cases and the presumptions or maxims associated with their resolution. These commonsense rules offer guidance toward the right thing to do and hold “generally and for the most part, but not absolutely” (Miller 1996b, 5).
A coherent body of past cases, their resolution, and the associated maxims emerge from this iterating process. This typology of past cases serves a purpose similar to that of case law for a judge or disease taxonomy for a physician. It has been described as a form of experience-based wisdom ( Jonsen and Toulmin 1988b). The task of creating a typology of cases must be recognized, however, as methodologically distinct from that of deciding cases by analogy. Moral taxonomies are formulated by applying theoretical ideas about what matters to the inductive classification of case-based experiences. In this way, applied moral reasoning is not possible without a guiding theory ( Jonsen 1991; Seay 2002). That is, it is not reasonable to ask “What defines a case of injustice?” without first identifying justice as a concern. When a new case arises, the ethicist (judge, doctor) identifies its salient features and critically deliberates its fit with paradigmatic cases in the typology. The degree of fit as well as the dimensions of misfit point to alternative paradigms and their associated moral obligations.
To the extent that a new case deviates from familiar paradigms, several implications may follow. First, the force of the maxim may be softened in that case. Second, competing paradigms may invoke competing maxims (which then must be reconciled with the usual agony familiar to anyone who has tried principle-based bioethical reasoning). But third, the misfit may produce such tension (e.g., fitting perfectly in one respect and not at all in another) that the moral framework—that is, the typology itself—must be adapted to accommodate the novel situation. Some would say, for example, that our standard ideas about humanity (and entailed rights, dignity, etc.) are being reformed by confrontations with cases of chimeric creatures that integrate human and animal genes (Baylis and Robert, forthcoming). New cases thus perpetuate the casuistic reasoning cycle and exert some influence on an evolving evaluative framework and classification scheme as they become part of the case history. New cases themselves can also become the precedents or paradigms for examining cases yet to come.
A Case-Based Model for Assessment and Decision Making
To summarize, there are three distinct phases of a case-based assessment and decision cycle: (A) taking stock and formulating precedents, (B) deciding cases, and (C) assimilating decisions. Figure 1 illustrates how such a casuistic, case-based reasoning might correspond to a cycle of decision making for emerging technologies. These phases unfold in order within an ongoing cycle: what occurs in the third phase influences how the first phase unfolds the next time around. How might this approach to decision making, adapted from casuistry and common law, correspond to policymaking regarding novel health technologies?
Taking Stock and Formulating Precedents. “Precedent” technologies are
derived from a knowledge of covered services (and services expressly refused
coverage). To determine whether a new technology fits, we need
a good sense of the contours of the current health system. Taking stock
involves not only inventorying the contents of the health system but
also interpreting what commitments these covered services signify. For
example, familiar themes are care of the elderly, concern for the infertile,
a dedication to prevention or lifesaving, solidarity with those suffering
rare problems, prudent value for money, ideas about what makes for
a “quality” life, stakeholders to be placated, providers to be humored,
private responsibility for “lifestyle” decisions, and so forth. We classify
existing services into the categories that we can agree are important. For
example, using the widely publicized typology developed for Oregon’s
Medicaid plan, Swedish decision makers assigned top priority to life-threatening,
treatable, and chronic disease; palliative care; and “care of
people with reduced autonomy” (Hjortsberg and Ghatnekar 2001, 28).
Second-place priority is held by preventive care and rehabilitation, third
by “less serious and acute chronic diseases,” and lowest priority by “care
for reasons other than disease or injury” (Hjortsberg and Ghatnekar
2001, 28). In the case-based reasoning cycle, such categories emerge
jointly from abstract ideas about what the health system “ought” to be
(perhaps applying a formal normative framework such as utilitarianism
and communitarianism, interpreting principles declared important in
policy documents or statutes, or adapting a framework used by another
jurisdiction) and an inductive interpretive exploration of the health system
as it “is.”
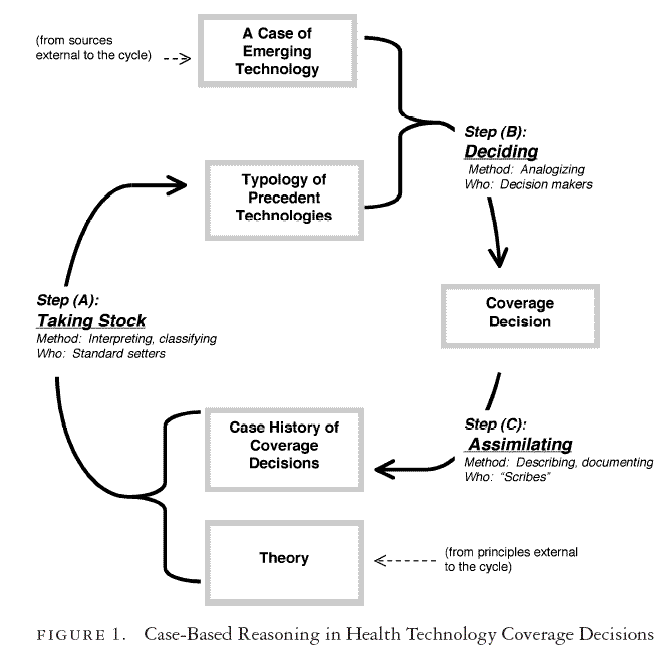
It is important that theory be brought into the cycle from the ethical or political milieu outside the cycle itself (dotted arrows in Figure 1). In this way, a focus on cases cannot liberate decision makers from the necessity of guiding evaluative principles ( Jonsen 1991, 1995a, 1995b; Wildes 2000). From the union of an organizing theory with a good body of cases to organize emerges a typology of services that both captures and conveys the spirit of our health system commitments. Examples of each class then become shorthand for key issues in coverage deliberations. These paradigms are like the technologies we instinctively invoke in policy arguments: for example, transplants and cardiac bypass surgery for their high costs or lifesaving ambitions, mammography for its wide promotion as a preventive or its association with women, prostate antigen screening for its ambiguous clinical value but countervailing association with men, early childhood education as a paradigmatic social determinant of health, and so forth. Those decision makers taking stock will be setting standards for the health care system and so must have the legitimacy, authority, and skills to do so. They must be able to interpret, adapt, and reconcile conflicting and multidimensional public commitments. They should represent the relevant community and be skilled at moral deliberation. Information about the spectrum of covered services is required, with special attention to the “margins” of the system. Finally, both inductive and deductive classification methods are necessary to knit commitments in principle with commitments in practice and to develop meaningful paradigm cases.
Deciding Cases. Coverage decision makers face emergent health technologies one at a time. Like principles, the candidate technologies emerge from dynamics beyond the coverage decision-making system itself (see Figure 1). The task in coverage deliberations is to apply paradigm technologies—now regarded as “precedents”—by analogy to the candidate technologies, as illustrated by the case studies of ICSI, Viagra, and predictive genetic tests. As with arguments from precedent in a court of law, much is at stake in identifying the spectrum of possible precedents, their fit with the case, and the coverage obligation that the fit (or lack of fit) implies. In the process of searching for, examining, and defending precedents, there is an interplay between abstract principles regarding what should be covered and concrete policy commitments regarding what is covered, and why. Deliberation should be critical and thorough. Desirable deliberative procedures and participants have been suggested elsewhere (Daniels and Sabin 1997; Gutmann and Thompson 2002), though in most jurisdictions, the reality falls short of these ideals. Decision makers require access to, and understanding of, the typology generated in the “taking stock” step (A). Both evaluative and descriptive evidence also are required to characterize the candidate technology adequately. To the extent that decisions are “evidence based,” it is at this point in the cycle that evidence plays its role. Crucial analytic methods include the arts of analogizing and distinguishing precedents (e.g., as developed in the profession of law).
Assimilating Decisions. New decisions must be incorporated back into policymakers’ understandings of the basic commitments—and limited obligations—represented by publicly insured health services. For example, in assessing Viagra as a “lifestyle” technology or ICSI as a “gender equity issue,” we may alter our understanding of these terms and their importance to judging future technologies. Daniels and Sabin (1997) highlight this type of assimilation as a strength of publicly accountable decision making: “One important effect of making public the reasons for coverage decisions is that, over time, the pattern of such decisions will resemble a type of ‘case law’. . . leading to more efficient, coherent, and fairer decisions over time” (Daniels and Sabin 1997, 327). When established, this feedback system maintains a kind of “institutional reflective equilibrium” (Daniels and Sabin 1997, 328), in which similar cases of new technologies are treated in the same way and defects in our moral reasoning about the value of health technologies are regularly recognized and addressed. Note that a new technology may, by analogy, throw doubt on the value of a paradigm technology and inspire a kind of housecleaning of covered services. This happened, for example, in the case of Viagra, for which 8 percent of U.S. health plans actually de-insured all previously insured services for sexual dysfunction as part of their policy decision not to cover Viagra (Titlow et al. 2000).
The case history evolves over time and offers opportunities to reinterpret and update the value system behind health care coverage decisions: “The earlier decision [to cover a new technology or not] reflects a commitment to act on the cited reasons and principles in future similar cases” (Daniels and Sabin 1997, 327, italics in original). Assimilation requires enduring institutional memory, as well as information systems that allow access to both rationales and decisions. The case history may also be informed by scanning deliberations, decisions, and analyses conducted in other jurisdictions. An accessible record of coverage decisions is not merely an administrative formality. It is an embodiment of policy precedents and the rules that they imply. As Schauer points out, precedent is commonly believed to “consist not only of the previous events and a decision, but also of the precedent court’s description of those facts. . . . The description of the precedent facts is in reality a generalization, and as a result this articulated description constitutes the factual predicate for a rule” (Schauer 1991, 185, italics in original). The obligation to give reasons helps to clarify policy commitments and to constrain future policymakers from making capricious decisions (Schauer 1995).
Conclusion
Health care technologies are evaluated comparatively. In traditional, evidence-based effectiveness and efficiency assessments, the closest clinical alternative often serves as the standard comparator. However, a much more diverse set of comparators is required to generate the arguments that resolve controversial coverage decisions at the level of publicly insured services. At this broader level of policymaking, decision makers invoke widely divergent technologies to highlight issues such as the needs of target populations, the medical versus social nature of effects, cost levels, and cost-effectiveness levels. Thus we end up with analyses that, for example, compare Viagra not with other interventions for erectile dysfunction but with lung transplants, mammography, hair restoration, or contraception. ICSI, too, is contrasted with heart/lung transplants, treatments for AIDS, hospital renovations, and so forth. Such ambitious comparisons are crucial to understanding the value of disorienting technologies.
Analogical comparisons of health care technologies serve several functions beyond weighing the instrumental usefulness of different technologies or asking how best to produce a life year. First, analogy is one of very few means we have for asking qualitative questions about the merits of technological uses, before moving on to quantitative questions about the degree of usefulness. Bioethicists use analogical comparisons extensively in their analyses of the ethical impacts of emerging technologies. Many promote case-based technology assessment as a promising way to address ethical dimensions of new technologies (Arras 1991; Daniels and Sabin 1997; Reuzel et al. 1999, 2001). Second, analogical reasoning gives force to all technology assessment criteria—whether effectiveness, cost-effectiveness, or purpose—by pointing decision makers to possible standards. While evidence may tell us “how good” a new service is, it cannot tell us how good is good enough. Already covered services serve as implicit standards of acceptability, and newly covered services set the tone for future coverage deliberations. There are other potential sources of more deductively derived standards (see, e.g., Hirth et al. 2000), but none is well developed and probably none is so frequently and naturally invoked as the ad hoc analogy to already covered health services.
Third, argument by analogy offers a means for playing evaluative criteria off one another. It has become common, for example, to promote cost-effectiveness as the single most important criterion by which to judge a new technology worthy of coverage or not. Yet we need only assemble a table showing the 20-fold superior cost-effectiveness of Viagra over lung transplants (Stolk et al. 2000) to inspire the question: What societal commitments and technological purposes besides the efficient production of QALYs distinguish these two technologies from each other? There ensues fruitful discussion of the needs of different target populations, the merits of different immediate clinical objectives, population health objectives, personal responsibility, the rule of rescue, and other concerns. In this way, arguments by analogy excel at “outing” implicit evaluative criteria, revealing their importance, and reconciling them into a more holistic picture of an emerging technology’s value. Finally, analogizing is a natural method of learning (Holyoak and Thagard 1995). Precedent offers coherence to policy development over time (Schauer 1987), and properly structured case-based deliberations about health technology coverage promise to continually improve our assessment skills (Daniels and Sabin 2002). The case-based reasoning cycle thus drives an important form of institutional learning.
There is a broad step to be taken, however, between technology assessment by analogy and coverage decision making by precedent. Precedent-based decision making requires not only rigorous analogical reasoning but also the institutional resources to develop, interpret, and apply precedent-based rationales consistently and credibly. If case-based reasoning is formalized and supported as a systematic method of technology assessment, case-by-case coverage decisions may yield wiser and more coherent packages of insurance benefits or entitlements. The case-based reasoning cycle outlined in Figure 1 points to the intellectual and institutional resources required. Each phase requires distinctive types of information, methods of analysis, and stripes of decision makers. If developed carefully, a case-based (or “case law”; Daniels and Sabin 1997) approach like this may support collective, systematic processes of deliberation that will generate more credible and defensible decisions.
To the limited extent to which a case-based approach has been advocated, there has been a tendency to focus on the right side of Figure 1, that is, the deliberative forum and evidence used to evaluate each case of new technology and to make the appropriate decision. In future analyses, we need more attention to what it means for a technology to “fit” into the health care system and, in particular, the role of precedent technologies as explicit or implicit standards. We also need more analytic attention to the left side of the cycle: how we assimilate discrete decisions into a global characterization of health system commitments and how we use covered services to exemplify proper policy approaches to new and unfamiliar services. The legitimate execution of step B (Figure 1) requires a methodical approach to steps A and C. In particular, we need to consider the problem of interpreting the legacy of past coverage decisions and judging what our currently covered services now stand for. Regardless of why specific technologies were covered in the first place, it matters a great deal how decision makers interpret the fact that they are covered now. Decision makers considering new technologies therefore must have a perspective on the old ones. For example, consider the questions that might reasonably arise in the context of predictive genetic tests: Do we currently cover genetic diagnostic services, and if so, which ones? If not, why not? Do we cover “all” diagnostic services, and if not, what have the traditional limits been? Do conventional diagnostics include risk assessments? Does this have blanket implications for the place of new molecular genetic tests emerging for hundreds of diseases? Or should we focus only on BRCA1/2 tests now and consider our level of commitment to cancer risk predictors in the context of the cancer care system? But will this have any bearing on future coverage of tests for predispositions or resistance to diabetes, heart disease . . . or infertility, or perhaps even erectile dysfunction?
To answer these cascading questions and to apply the answers to defensible coverage decisions, we need certain resources. Intellectual resources include skill at analogizing, a method that might be developed by importing knowledge from psychology, philosophy, political science, law, and ethics. Other intellectual demands include the ability to take account of and assimilate old decisions and to classify and reclassify covered services into meaningful categories. Furthermore, theoretical principles— from outside the immediate casuistic reasoning cycle—are required to guide this classification. Although this ethically unfashionable and uncomfortable requirement may be obscured, ignored, or met poorly, it cannot really be avoided. Prescriptive ideas about what is worthy in the health care system cannot emerge entirely from a descriptive inventory of what is there. Professed values and goals of the health care system must be kept in focus and related faithfully to this framework.
Information resources also are needed. Evaluative evidence is, of course, important; effectiveness and cost-effectiveness count in nearly every coverage decision-making scheme. Equally important—especially to technologies we experience as “challenging”—are various visions of the means and ends of a given technology’s use, as well as ideas about how impacts might be controlled through, for example, regulation, markets, conditional coverage, guidelines, or other policy instruments (Giacomini, Miller, and Browman 2002). Institutions are required to carry out the information gathering, reasoning, deciding, and rationalizing. The entire casuistic cycle could be conducted by one body (e.g., within a health plan) or could be divided among standard setters (step A), decision makers (step B), and “scribes” (step C). These could involve different types of decision makers and deliberative processes and might require different levels of expert, bureaucratic, and public involvement to be both effective and legitimate. Communication and relationships of authority and accountability among the groups need additional specification and care. The ideal, as well as the feasible, institutional arrangements vary with the jurisdiction. In particular, publicly funded health plans (e.g., Canadian medicare, U.S. Medicare) operate under different incentives and obligations than do private health plans (e.g., in the United States). These differences affect the moral force of precedent-based arguments as well as the framework of values within which precedent-based rationales are formulated.
Formalizing this analytic process may seem laborious. It is essential,
however, to recognize and respect precedent-based reasoning for what
it is: a pervasively practiced form of health technology assessment, especially
in the context of immediate coverage deliberations. It is one
of the few means that decision makers currently have for establishing
standards, evaluating the qualitative and ethical dimensions of new technologies,
and reconciling normative evaluation criteria (purpose, equity,
etc.) with instrumental ones (effectiveness, efficiency, etc.). We already
do it informally as we struggle with coverage decisions. We should begin
to think of arguments from analogy as an evaluative methodology that
offers much insight but also requires skill and support to do well.
References
Akanmidu, R.A. 2001. The Morality of Precedent in Law. Ratio Juris 14(2):244–51.
Aronovitch, H. 1997. The Political Importance of Analogical Argument. Political Studies 45:78–92.
Arras, J.D. 1991. Getting Down to Cases: The Revival of Casuistry in Bioethics. Journal of Medicine and Philosophy 16:29–51.
Atkinson, T. 2002. Lifestyle Drug Market Booming. Nature Medicine 8(9):909.
Banks, C.P. 1999. Reversals of Precedent and Judicial Policy-Making: How Judicial Conceptions of Stare Decisis in the U.S. Supreme Court Influence Social Change. Akron Law Review 32(2):233–58.
Baylis, F., and J.S. Robert. (forthcoming) Radical Rupture: Exploring Biological Sequelae of Germ-Line Genetic Intervention. In A Dividing Line? Toward an Ethics of Germline Gene Therapy, edited by J.E.J. Rasko, G.M. O’Sullivan, and R.A. Ankeny. Cambridge: Cambridge University Press.
Calltorp, J. 1999. Priority Setting in Health Policy in Sweden and a Comparison with Norway. Health Policy 50(1–2):1–22.
Cameron and Smith v. Nova Scotia. 1999. Nova Scotia Court of Appeal.
Commission on the Future of Health Care in Canada. 2002. Building on Values: The Future of Health Care in Canada. Saskatoon.
Copi, I.M. 1986. Analogy. In Informal Logic, by I.M. Copi, 167–96. New York: Macmillan.
Creinin, M.D., R. Lisman, and R.C. Strickler. 1999. Screening for Factor V Leiden Mutation before Prescribing Combination Oral Contraceptives. Fertility and Sterility 72(4):646–51.
Daniels, N., and J.E. Sabin. 1997. Limits to Health Care: Fair Procedures, Democratic Deliberation, and the Legitimacy Problem for Insurers. Philosophy and Public Affairs 4:303–50.
Daniels, N., and J.E. Sabin. 2002. Accountability for Reasonableness. In Setting Limits Fairly; Can We Learn to Share Medical Resources? by N. Daniels and J.E. Sabin, 43–66. New York: Oxford University Press.
Daniels, N., J.R. Teagarden, and J.E. Sabin. 2003. An Ethical Template for Pharmacy Benefits. Health Affairs 22(1):125–37.
Deber, R., E. Ross, and M. Catz. 1994. Comprehensiveness in Health Care: Report to the HEAL Action Lobby. Toronto: University of Toronto, Department of Health Administration.
D.R. and L.R., B.C. and L.A.C., B.L. and R.F., L.E. and M.E., J.H. and K.H. v. Ontario. 1998. Ontario Health Services Appeal Board.
Eisenhower, J.J. III. 1988. Four Theories of Precedent and Its Role in Judicial Decisions. Temple Law Review 61:871–7.
Fletcher, C.E. III. 1988. Judicial Dialectics. Cumberland Law Review 19(1):97–197.
Giacomini, M., F. Miller, and G. Browman. 2002. Confronting the “Grey Zones” of Technology Assessment: Evaluating Genetic Testing Services for Public Insurance Coverage in Canada. International Journal of Technology Assessment in Health Care 19(2):301–15.
Government Committee on Choices in Health Care. 1992. Choices in Health Care. Zoetermeer (Netherlands): Ministry of Welfare, Health and Social Affairs.
Grann, V.R., W. Whang, J.S. Jacobson, D.F. Heitjan, K.H. Antman, and A.I. Neugut. 1999. Benefits and Costs of Screening Ashkenazi Jewish Women for BRCA1 and BRCA2. Journal of Clinical Oncology 17(2):494–500.
Gutmann, A., and D. Thompson. 2002. Just Deliberation about Health Care. In Ethical Dimensions of Health Policy, edited by M. Danis, C. Clancy, and L.R. Churchill, 77–96. New York: Oxford University Press.
Hansson, L., O. Norheim, and K. Ruyter. 1994. Equality, Explicitness, Severity, and Rigidity: The Oregon Plan Evaluated from a Scandinavian Perspective. Journal of Medicine and Philosophy 19:343–66.
Haslam, D. 1998. Viagra and Penicillin: The Same Problem? The Practitioner 242:725.
Hirth, R.A., M.E. Chernew, E. Miller, A.M. Fendrick, and W.G. Weissert. 2000. Willingness to Pay for a Quality-Adjusted Life Year: In Search of a Standard. Medical Decision Making 20(3):332–42.
Hitchcock, D. 1992. Reasoning by Analogy: A General Theory. In The Generalizability of Critical Thinking: Multiple Perspectives on an Educational Ideal, edited by S.P. Norris, 109–24. New York: Teachers College Press.
Hjortsberg, C., and O. Ghatnekar. 2001. Health Care Systems in Transition: Sweden. European Observatory on Health Care Systems 3(8):1–97.
Hofstadter, D. 1996a. Fluid Concepts and Creative Analogies: Computer Models of the Fundamental Mechanisms of Thought. New York: Basic Books.
Hofstadter, D. 1996b. Variations on a Theme as the Crux of Creativity. In Metamagical Themas: Questing for the Essence of Mind and Pattern, by D. Hofstadter, 232–59. New York: Basic Books.
Holyoak, K.J., and P. Thagard. 1995. Mental Leaps: Analogy in Creative Thought. Cambridge, Mass.: MIT Press.
Hurley, J., J.L. Cosby, M. Giacomini, and B. Hutchison. 2000. Making Resource Allocation Decisions in the Health Care Sector: A Review of Some Recent Proposals. Saskatoon: HEALNet Regionalization Research Centre.
Jonsen, A.R. 1991. Of Balloons and Bicycles, or, the Relationship between Ethical Theory and Practical Judgment. Hastings Center Report, September/October, 14–16.
Jonsen, A.R. 1995a. Casuistry: An Alternative or Complement to Principles? Kennedy Institute of Ethics Journal 5(3):237–51.
Jonsen, A.R. 1995b. The Weight and Weighing of Ethical Principles. In The Ethics of Research Involving Human Subjects: Facing the 21st Century, edited by H.Y. Vanderpool, 59–82. Frederick, Md.: University Publishing Group.
Jonsen, A.R., and S. Toulmin. 1988a. The Abuse of Casuistry. Berkeley: University of California Press.
Jonsen, A.R., and S. Toulmin. 1988b. The Achievement of Casuistry. In The Abuse of Casuistry, by A.R. Jonsen and S. Toulmin, 250–65. Berkeley: University of California Press.
Keith, A. 2000. The Economics of Viagra. Health Affairs 19(2):147–57.
King, C.H., D.F. Fischler, and R.D. Gerkin. 2002. Will Genetic Testing Alter the Management of Disease Caused by Infectious Agents? A Cost-Effectiveness Analysis of Gene-Testing Strategies for Prevention of Rheumatic Fever. Clinical Infectious Diseases 34(11):1491–9.
Kwok, Y.S., and C. Kim. 1999. Valuing Viagra: What Is Restoring Potency Worth? Effective Clinical Practice 2(4):171–5.
Miller, R.B. 1996a. Casuistry and Modern Ethics. Chicago: University of Chicago Press.
Miller, R.B. 1996b. Casuistry, Politics, and Moral Complexity. In Casuistry and Modern Ethics, by R.B. Miller, 3–16. Chicago: University of Chicago Press.
Parens, E. 1998. An Immodest Proposal. Hastings Center Report 28(3):44.
Phillips, K.A., J. Spetz, and J.S. Haas. 2003. Viagra and Contraceptives. Health Affairs 22(1):277.
Reuzel, R.P.B., G.J. van der Wilt, H.A.M.J. ten Have, and P.F de Vries Robbe. 1999. Reducing Normative Bias in Health Technology Assessment: Interactive Evaluation and Casuistry. Medicine, Health Care and Philosophy 2:255–63.
Reuzel, R.P.B., G.J. van der Wilt, H.A.M.J. ten Have, and P.F de Vries Robbe. 2001. Interactive Technology Assessment and Wide Reflective Equilibrium. Journal of Medicine and Philosophy 2001(3):245–61.
Rifkin, J. 2001. Precedent Is “Double Sided.” Amherst, Mass.: University of Massachusetts Press.
Schauer, F. 1987. Precedent. Stanford Law Review 39:571–605.
Schauer, F. 1991. Rules and Law. In Playing by the Rules: A Philosophical Examination of Rule-Based Decision-Making in Law and in Life, by F. Schauer, 167–206. Oxford: Clarendon Press.
Schauer, F. 1995. Giving Reasons. Stanford Law Review 47:633–59.
Schlesinger, M., and R. Lau. 2000. The Meaning and Measure of Policy Metaphors. American Political Science Review 94(3):611–26.
Seay, G. 2002. Theory Skepticism and Moral Dilemmas. Kennedy Institute of Ethics Journal 12(3):279–98.
Senate Standing Committee on Social Affairs Science and Technology. 2002. Recommendations for Reform. Vol. 6 of The Health of Canadians—The Federal Role. Ottawa: Thirty-seventh Parliament of Canada.
Smith, K.J., and M.S. Roberts. 2000. The Cost-Effectiveness of Sildenafil. Annals of Internal Medicine 132(12):933–7.
Stolk, E.A., J.J. Busschbach, M. Caffa, E.J. Meuleman, and F.F. Rutten. 2000. Cost Utility Analysis of Sildenafil Compared with Papaverine-Phentolamine Injections. British Medical Journal 320(7243):1165–8.
Stone, D. 1998. Policy Paradox: The Art of Political Decision Making. New York: Norton.
Sunstein, C.R. 1993. Commentary: On Analogical Reasoning. Harvard Law Review 106:741–91.
Titlow, K., L. Randel, C.M. Clancy, and E.J. Emanuel. 2000. Drug Coverage Decisions: The Role of Dollars and Values. Health Affairs 19(2):240–7.
Wildes, K. 2000. After Paradigms: The Crisis of Secular Casuistry. In Moral Acquaintances: Methodology in Bioethics, by K. Wildes, 86–121. South Bend, Ind.: University of Notre Dame Press.
Wildhagen, M.F., H.B. Hilderink, J.G. Verzijl, J.B. Verheij, L. Kooij, T. Tijmstra, L.P. ten Kate, and J.D. Habbema. 1998. Costs, Effects, and Savings of Screening for Cystic Fibrosis Gene Carriers. Journal of Epidemiology and Community Health 52(7):459–67.
Wilson, R., M.S. Rowan, and J. Henderson. 1995. Core and Comprehensive
Health Care Services: 1. Introduction to the Canadian Medical
Association’s Decision-Making Framework. Canadian Medical Association
Journal 152:10636.
Acknowledgments: I thank the following colleagues for their comments and suggestions
regarding work in progress: Nuala Kenny, Jeremiah Hurley, Françoise
Baylis, Josephine Johnston, Jocelyn Downie, Susan Sherwin, Susan Goold, Fiona
Miller, and the late Bernie O’Brien. I also am grateful to three anonymous reviewers
and to Brad Gray for their helpful suggestions. Jason Sigurdson, Lydia
Garland, and Deirdre DeJean provided research assistance. I was supported during
this project by a Picchione Visiting Scholar Award from the Dalhousie
Medical Research Foundation, a grant from the Nova Scotia Health Research
Foundation, and a Scholar Award and Short Term Exchange Grant from the
Canadian Institutes for Health Research. This work also benefited from the
support and collegiality of the Department of Bioethics at Dalhousie University,
the Novel Genetic Technologies Research Group at Dalhousie University, and
the Centre for Health Economics and Policy Analysis at McMaster University.
Address correspondence to: Mita Giacomini, Centre for Health Economics and Policy Analysis, Department of Clinical Epidemiology & Biostatistics, McMaster University, 1200 Main Street West, HSC–3H1C, Hamilton, Ontario L8N 3Z5, Canada (e-mail: giacomin@mcmaster.ca).
(c) 2005 Milbank Memorial Fund
The Milbank Memorial Fund is an endowed operating foundation that engages in nonpartisan analysis, study, research, and communication on significant issues in health policy. In the Fund's own publications, in reports or books it publishes with other organizations, and in articles it commissions for publication by other organizations, the Fund endeavors to maintain the highest standards for accuracy and fairness. Statements by individual authors, however, do not necessarily reflect opinions or factual determinations of the Fund.
This file may be redistributed electronically or in print as long as it remains wholly intact, including this notice and copyright. The Fund will freely distribute this document in its original published form on request.
Online producer: Stephanie Moe-Quiggle